
世界卫生组织HVAC非无菌制药指南(中英对照)WHO-937
53页1、WHO 937 Annex 附件2Supplementary guidelines on good manufacturing practices for heating, ventilation and air-conditioning systems for non-sterile pharmaceutical dosage forms在GMP的基础上补充指导药剂生产的供暖,通风,空调系统的指导条例。1. Introduction 介绍2. Scope of document 文本范围3. Glossary 术语表4. Protection 保护4.1 Products and personnel 产品和人员4.2 Air filtration 空气过滤4.3 Unidirectional airflow 单向空气流4.4 Infiltration 渗透4.5 Cross-contamination 交叉污染4.6 Temperature and relative humidity 温度和相对湿度5. Dust control 固体颗粒污染物控制6. Protection of t
2、he environment 环境保护6.1 Dust in exhaust air 排气中的固体颗粒6.2 Fume removal 烟尘的祛除7. Systems and components 系统和构造7.1 General 概论7.2 Recirculation system 循环系统7.3 Full fresh air systems 送风系统8. Commissioning, qualification and maintenance 运转,资格和维护8.1 Commissioning 运转8.2 Qualification 资格8.3 Maintenance 维护References 文献451. Introduction 介绍Heating, ventilation and air-conditioning (HVAC) play an important role in ensuring the manufacture of quality pharmaceutical products. A well designed HVAC system will also p
3、rovide comfortable conditions for operators.加热,通风,空调(HVAC)系统在保证药品质量的生产上起着至关重要的作用。同时,设计完善的系统也会给操作人员提供舒适的工作环境。These guidelines mainly focus on recommendations for systems for manufacturers of solid dosage forms. The guidelines also refer to other systems or components which are not relevant to solid dosage form manufacturing plants, but which may assist in providing a comparison between the requirements for solid dosage-form plants and other systems.这些条例主要是针对固体药剂生产车间而提出的。但同时在涉及到与固体药剂生产无关的其它生产中,这些条
4、例可以比较固体药剂生产车间和其它车间生产规格上的具体要求。HVAC system design infl uences architectural layouts with regard to items such as airlock positions, doorways and lobbies. The architectural components have an effect on room pressure differential cascades and cross-contamination control. HVAC系统的设计与建筑规划有关,比如气阀、门口、出入口。这些物件的设计对房间气压差的形成和交叉污染的控制都起着重要作用。The prevention of contamination and cross-contamination is an essential design consideration of the HVAC system.污染和交叉污染的防制是HVAC系统设计的重点考虑因素。 In view of these critical aspec
《世界卫生组织HVAC非无菌制药指南(中英对照)WHO-937》由会员人***分享,可在线阅读,更多相关《世界卫生组织HVAC非无菌制药指南(中英对照)WHO-937》请在金锄头文库上搜索。

产业暨文化旅游节活动医疗保障方案范文(二篇)

大班上学期科学教案《会转的花》.doc

大学生毕业晚会的策划书范文.doc

主任述职报告锦集9篇

闲置废旧高炉等设备②拆除工程

汽车机械基础课程标准(新)

IPMA人力资源战略与规划课后习题整理

湖南省义务教育实验教科书四年级下科技教案

2023年保险公司工作总结5篇(年保险公司工作总结个人)

现场改善的基本方法--问题解决七步法

数控机床维修大赛考试试题gfqu

劳务协议简易经典版(五篇).doc

某汽车公司对供应商要求的文件-大力推荐(1)

2023年五年级班主任年度工作总结报告3篇(班主任学期工作总结年小学)

八年级数学上册第12章整式的乘除12.1幂的运算12.1.3积的乘方导学案新版华东师大版

重阳节联谊会策划方案-精选模板

2023年竞选副大队长演讲稿5篇竞选副大队长的竞选稿

某某餐饮集团店长操作新手册
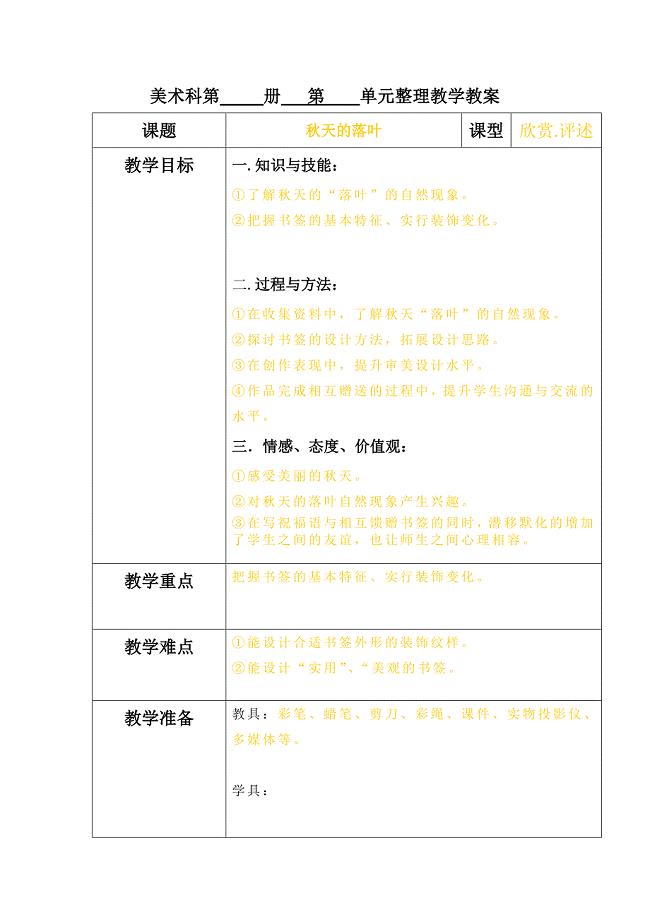
《秋天的落叶》教案()

..卷扬机安全操作规程-
 年人教版新目标八年级上 Unit6 单元测试试卷及答案
年人教版新目标八年级上 Unit6 单元测试试卷及答案
2022-11-13 9页
 健康过冬从护理鼻腔开始
健康过冬从护理鼻腔开始
2023-02-27 7页
 单病种管理汇总
单病种管理汇总
2024-02-09 62页
 高中数学人教B版必修4:双基限时练含解析双基限时练【12】
高中数学人教B版必修4:双基限时练含解析双基限时练【12】
2022-11-21 10页
 北师大版八年级数学下册分式名师讲义含答案
北师大版八年级数学下册分式名师讲义含答案
2023-05-26 3页
 移动互联网背景下体育健身类App地现状及对策研究
移动互联网背景下体育健身类App地现状及对策研究
2022-07-20 18页
 基于单片机控制的交通灯设计论文含完整程序原理图
基于单片机控制的交通灯设计论文含完整程序原理图
2023-08-09 43页
 几年技术工作总结
几年技术工作总结
2022-12-02 33页
 八年级数学上册第12章整式的乘除12.1幂的运算12.1.3积的乘方导学案新版华东师大版
八年级数学上册第12章整式的乘除12.1幂的运算12.1.3积的乘方导学案新版华东师大版
2023-07-11 3页
 高中湘教版 地理必修3检测:章末高效整合3 Word版含解析
高中湘教版 地理必修3检测:章末高效整合3 Word版含解析
2023-12-27 7页

