
PDA-数据完整性行为守则要素
30页1、PDA-数据完整性行为守则要素(中/英对照版)Parenteral Drug Association Points to Consider注射剂协会考虑要点Elements of a Code of Conduct for Data Integrity数据完整性行为守则要素Introduction简介Data Integrity has been and currently is a major global concern of Health Authorities and the pharmaceutical industry.Although not a new issue, numerous recent Health Authority enforcement actions such as Warning Letters, Import Alerts, Product Detentions, and suspension or revocation of Marketing Authorizations has focused attention on Data Integ
2、rity.Data Integrity can result from lack of awareness of regulatory requirements, employee errors, failure to check accuracy of data, software or system malfunction, or configuration problems with electronic data handling, or malfeasance by employees.To holistically address Data Integrity, the Parenteral Drug Association (PDA) is developing a set of tools in the form of PDA Technical Reports, PDA Training Program, Data Integrity Workshops, and Points to Consider documents that can be used by ind
3、ustry to address this serious issue.This document presents the views of the Parenteral Drug Association (PDA) on the benefits for companies to voluntarily adopt a Code of Conduct for assuring data integrity.数据完整性目前已经成为全球卫生机构与制药行业所关注的重点。尽管不是一个新的问题,近期在很多卫生当局的执法行动例如警告信、进口警报、产品扣留,以及暂时取消或撤销上市许可中都非常关注数据完整性的问题。数据完整性可能由于缺乏法规要求意识、员工错误、无法核实数据准确性,软件或系统故障,或电子数据处理配置问题,或员工渎职行为导致。为了全面解决数据完整性问题,注射剂协会(PDA)正在开发一套面向行业的涵盖PDA技术报告、PDA培训程序、数据完整性研讨会,以及考虑要点文件的工具来解决这一严重问题。本文件代表注射剂协会(PDA)的观点,公司可自愿采用行为守则来确保数据完整性。How to Use
4、 this Document如何使用本文件This document was developed by a team with expertise in the fields of quality, regulatory affairs, auditing, and manufacturing and reviewed by attorneys specialized in food, drug and labor law. This document is written for easy adoption, in part or in its entirety, by companies, if they so choose, without the need for extensive rewriting of the document. Therefore, the terms shall and must have been used to permit the Code to be enforceable by a company, if adopted. This doc
《PDA-数据完整性行为守则要素》由会员热****分享,可在线阅读,更多相关《PDA-数据完整性行为守则要素》请在金锄头文库上搜索。

2022年二级建造师习题 施工管理
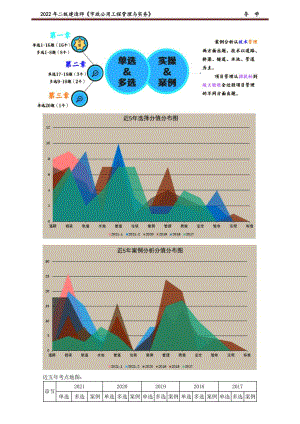
2022年JG二建市政习题班讲义 导学
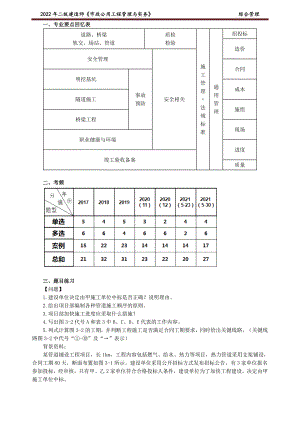
2022年JG二建市政习题班讲义综合管理

2022年JG二建市政基础班讲义 生活垃圾填埋处理工程

2022年JG二建市政基础班讲义 施工测量与监控量测
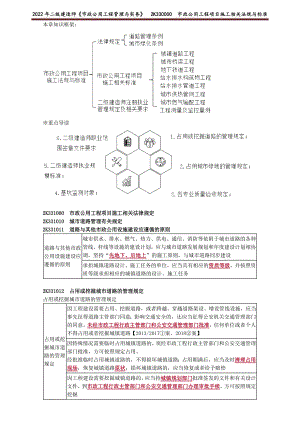
2022年JG二建市政基础班讲义 市政公用工程项目施工相关法规与标准
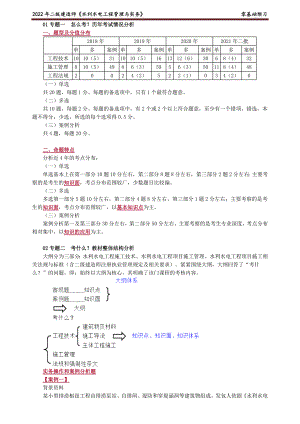
2022年二级建造师水利零基础预习

施工进度计划的类型及其作用

公路工程施工现场临时工程管理
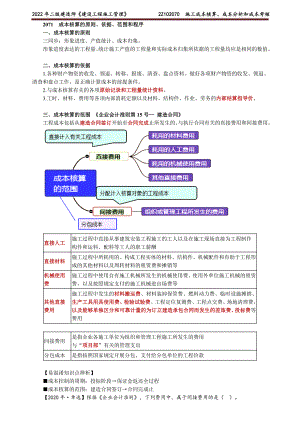
2022年二建管理精讲 施工成本核算、成本分析和成本考核

公路工程施工机械设备的使用管理
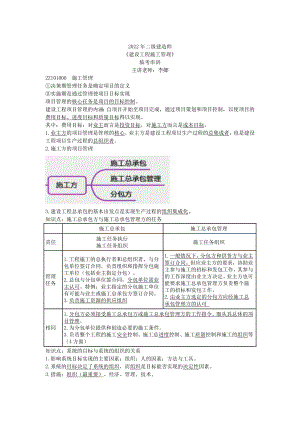
2022二建施工管理临考串讲

2022年二建管理精讲 职业健康安全管理体系与环境管理体系
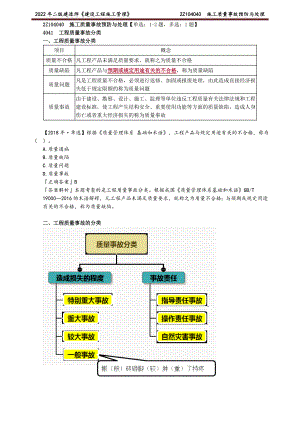
2022年二建管理精讲 施工质量事故预防与处理
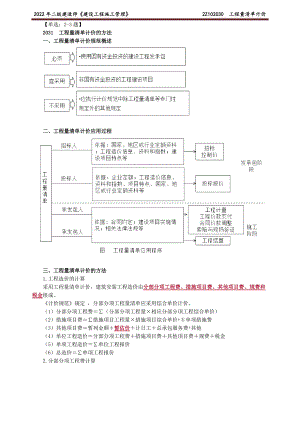
2022年二建管理精讲 工程量清单计价
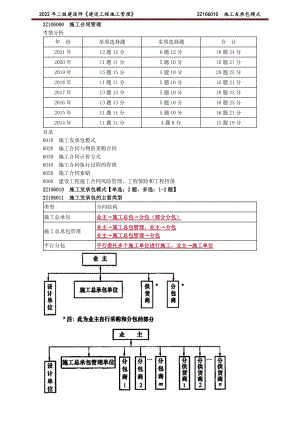
2022年二建管理精讲 施工发承包模式

二级建造师(机电工程)注册执业管理规定及相关要求
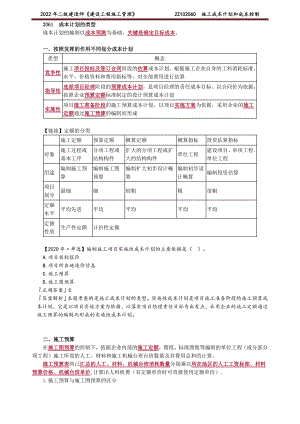
2022年二建管理精讲 施工成本计划和成本控制

2022年二建管理精讲 施工质量控制的内容和方法

隧道围岩分级与隧道构造
 《工程数据分析》-课程教学大纲
《工程数据分析》-课程教学大纲
2024-01-10 5页
 郑州市某公司多层现浇钢筋混凝土框架结构办公楼设计
郑州市某公司多层现浇钢筋混凝土框架结构办公楼设计
2023-11-12 92页
 人工智能未来发展和技术创新
人工智能未来发展和技术创新
2023-09-04 3页
 AI人工智能时代:个人如何应对时代变化
AI人工智能时代:个人如何应对时代变化
2023-09-04 2页
 AI大模型资源和设计模型
AI大模型资源和设计模型
2023-09-04 2页
 e趣购app的设计与实现毕业设计
e趣购app的设计与实现毕业设计
2023-08-29 51页
 乡机勃勃智慧农场互动云平台的设计与实现毕业设计
乡机勃勃智慧农场互动云平台的设计与实现毕业设计
2023-08-29 53页
 大语言模型及代码
大语言模型及代码
2023-08-14 4页
 常见的Vue面试题及答案
常见的Vue面试题及答案
2023-08-14 5页
 程序员简历模板-精美面试模板(墙裂推荐)
程序员简历模板-精美面试模板(墙裂推荐)
2023-08-14 1页

