
GMP偏差处理中英文
19页1、1.PURPOSE 目的Whenever a product, material or system fails to meet the specifications or in the event of a failure to comply with relevant documentation or regulatory requirements, an appropriate investigation must be undertaken, the cause(s) identified and the necessary corrective actions taken 当产品、物料或系统不符合质量标准要求或某事件不符合相关文件或法规要求时, 必须进行适当的调查,查明原因并采取必要的改正措施。2. SCOPE 范围This SOP covers all failures and unplanned incidents related to Chemical components, Packaging materials, Drug Products, Processes,
2、Systems, Equipments, Utilities and Facilities used to produce and control them.本 SOP 适用于处理所有失误及非计划性故障事件,含概用于产品并控制产品的化学成分、包装材料、药品、工艺、系统、设备、公共设施和厂房等。3. DEFINITIONS/ABBREVIATIONS 定义/缩写Deviation ( also known as anomaly ) : Any unplanned change from a written procedure/document, during manufacturing or testing or a non-conformance to approved specifications or any failure on GMP-related systems. Deviations are assessed according to compliance and /or the risk they present to patient health and/or wit
3、h regulatory requirements. Deviations are to be classified as “critical or major or minor ” 偏差(通常也称为异常) : 在药品生产过程中,任何与既定的程序、文件不符的非计划的变更或 与批准的质量标准不符,或与GMP 相关的系统失败。偏差按照对患者造成的风险何国家法规的符合性进行评估。偏差可分为三类“严重偏差、主要偏差、微小偏差”Critical Deviations: 严重偏差:Critical deviations require immediate investigation, root cause analysis and corrective-preventive action. 严重偏差需要立即进行调查,查找问题的根本原因并制定纠正预防措施。A deficiency in material, drug product, medical device, system or service that can affect significantly the quality, purity,
4、 safety or efficacy of a product/medical device or can lead to health threatening conditions in drug product, or medical device. Alternatively, any deficiency that can lead to a non-compliant drug product/medical device or to a situation that may be cited by regulatory authorities as critical. 存在物料、 产品、 医疗器械或任何系统、 维护方面能严重影响产品质量、 纯度、 安全、 功效, 能对产品或身体健康产生危害的缺陷; 或者会导致产品质量不符合, 或可能被 法规部门视为严重缺陷项的缺陷;Major Deviation: 主要偏差:Major deviations require investigations, root cause analysis and corrective-prevent
《GMP偏差处理中英文》由会员公****分享,可在线阅读,更多相关《GMP偏差处理中英文》请在金锄头文库上搜索。

福州晶体硅生长设备研发项目可行性研究报告【参考模板】

八年级英语下册《第十单元》综合检测 人教新目标版

教育心理学笔记(记忆要点)
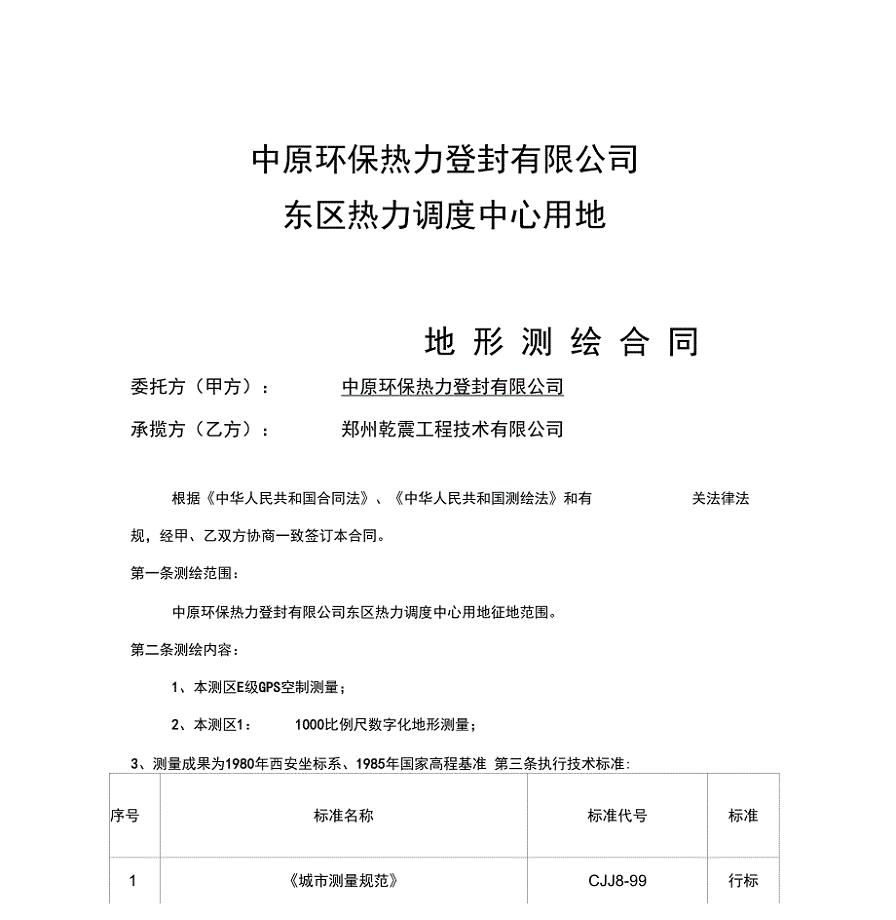
地形测绘合同

内部控制信息系统用户管理制度

2022年实用的婚礼主持词模板集锦8篇

关于春节的周记

联合投标合作协议书

商品混凝土行业成本构成分析
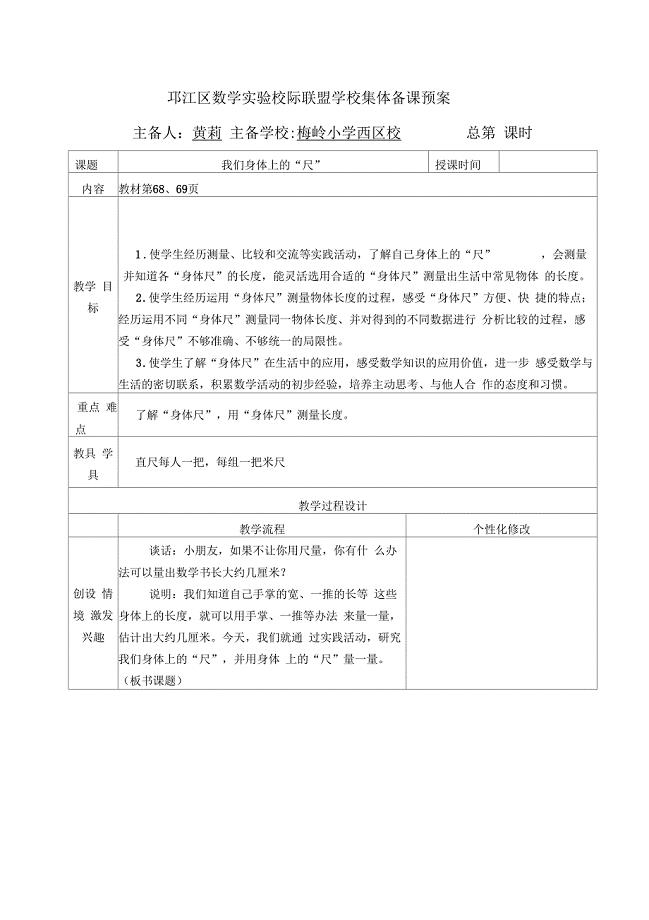
苏教版二上第五课时《我们身体上的“尺”》

五年级旋转教案

解直角三角形 (2)

AA林业局开展严厉打击非法占用林地专项整治活动

2022年福建省安管人员ABC证【官方】考试题库35含答案

Recycle1单元教案1

经理助理个人年终工作总结标准模板(3篇).doc

贵州地质概况

话题复习专练-自然与环境(一)B1u4-B6u5earthquke-the-power-of-nature)

清除校园牛皮癣活动参考策划书.doc

中小学生社团建设工作意见
 阅读成就你我的英语作文50字doc
阅读成就你我的英语作文50字doc
2023-09-22 5页
 2016期末英语教研组质量分析报告
2016期末英语教研组质量分析报告
2022-12-02 5页
 英语六级考试听力场景词汇完整版解析
英语六级考试听力场景词汇完整版解析
2023-10-17 10页
 牛津版5A——6A词组归纳
牛津版5A——6A词组归纳
2022-11-22 10页
 十大疯狂英语单词背诵方法
十大疯狂英语单词背诵方法
2024-02-19 3页
 密九语法180题和答案
密九语法180题和答案
2023-11-10 21页
 诚毅学院大学英语3重修材料(含答案)
诚毅学院大学英语3重修材料(含答案)
2022-09-10 28页
 英语高效课堂研修总结(一)
英语高效课堂研修总结(一)
2024-01-27 3页
 上班族学英语-分享你们一些提高效率的经验
上班族学英语-分享你们一些提高效率的经验
2023-10-24 9页
 如何从零开始学好英语
如何从零开始学好英语
2022-12-22 4页

