
江苏恒瑞医药股份有限公司的药品质量标准分析方法验证qc827b49
14页1、江苏恒瑞医药股份JIANGSU HENGRUI MEDICINE CO., LTD.管理文件和管理程序Administration Document & Management Procedure质量控制Quality Control 文件名:File Name:药品质量标准分析方法验证 Validation of analytical method adopted in pharmaceutical quality specification 文件号:QC-827BFile Code:页 号:Page 1 of 7 Pages:分发部门:Distribution 质量部 Quality department 执行日期:Executive date:2005年8月15日变更记载/History文件名称:File Name:文件号:File Code:执行日期:Implement Date变更原因:Rationale:药品质量标准分析方法验证QC-827B2005-08-15版面格式与总公司统一起草/日期Written By/Date审核/日期Reviewed By/Date批准/日期Ac
2、cepted By/DateQC-827B Page 2 of 71.目的 Purpose 证明采用的方法适合于相应检测要求。To testify the adopted methods meet corresponding detection requirements. 2.范围 Scope 适用于鉴别试验,杂质定量或限度检查,原料或制剂中有效成分含量测定,制剂中其它成分(如降解产物、防腐剂等)的测定。药品溶出度、释放度等功能检查中的溶出量等测试方法,以及微生物限度、细菌内毒素、无菌、清洗验证中的检验方法。It is applicable to identification; quantification or limit test of impurities; content determination of the active ingredient in drug substance or preparation and that of other components (degradation products, antiseptics etc. ) in the prepa
3、ration; dissolution and release test of pharmaceuticals; microbial limit test; tests for bacterial endotoxin, sterility; and test of cleaning validation. 3.程序 Procedures 化学检验方法 Chemical examination methods准确度 Accuracy 准确度系指用该方法测定的结果与真实值或参考值接近的程度,一般以回收率(%)表示。The accuracy of an analytical method is the closeness of test results obtained by that method to the true value or the reference value. Accuracy is often expressed as percent recovery value. 1. 含量测定方法的准确度Accuracy of the method for content det
4、ermination 原料药可用已知纯度的对照品或样品进行测定,或用本法所得结果与已建立准确度的另一方法测定的结果进行比较。The accuracy for drug substance may be determined with a reference substance or sample with known purity, or by comparing the result obtained by this method with the result obtained by another method of which the accuracy has been established. 制剂可用含已知量被测物的各组分混合物进行测定,或向制剂中加入已知量的被测物进行测定,或与另一已建立准确度的方法比较结果。The accuracy for drug preparation may be determined with the mixture of components, to which known amounts of analyte have been added.
《江苏恒瑞医药股份有限公司的药品质量标准分析方法验证qc827b49》由会员桔****分享,可在线阅读,更多相关《江苏恒瑞医药股份有限公司的药品质量标准分析方法验证qc827b49》请在金锄头文库上搜索。

某公司财务管理方案实用制度

联络部个人工作总结模板(2篇).doc

小学一年级班级管理工作总结(10篇)

人教版高中生物必修2课后习题参考答案

开幕式观后感

城市桥梁工程

致辞讲话致辞

XX食品集团的工作说明书

银行从业资格考试试题

2023年公司人事档案管理制度篇

哈尔滨植物免疫激活蛋白产品项目商业计划书

《钢铁是怎样炼成的》读后感1000字作文 - 小学生作文

车辆质押借款合同参考范本(7篇)
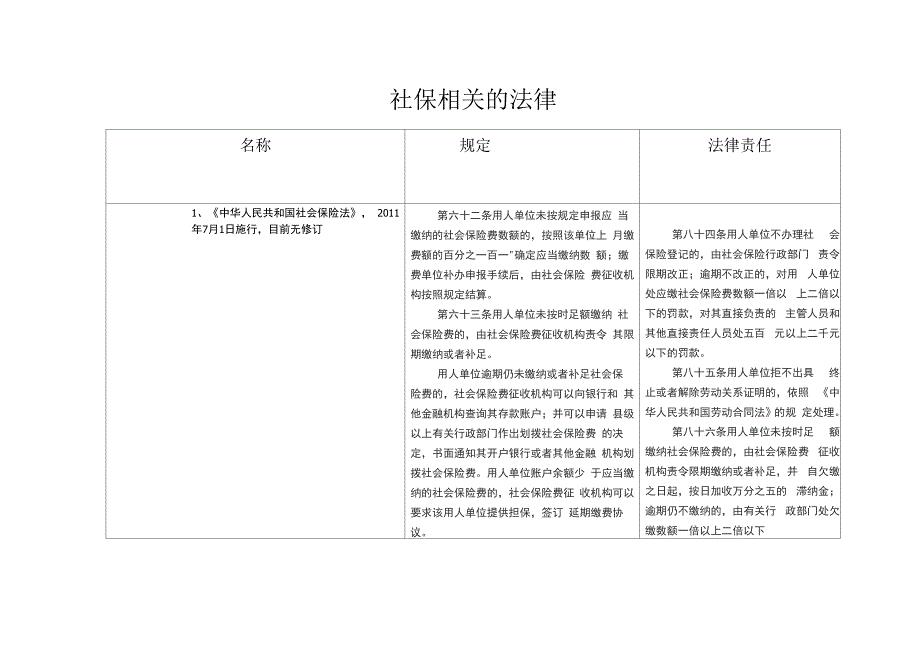
社保相关法律、行政法规、政策

人教部编版一年级语文上册第七单元知识点复习

丁座草苗木供货合同

海洋婚礼主持词

2023年吉林省四平市双辽市辽北街道北宁社区工作人员考试模拟试题及答案

儿童摄影合作协议范本新版

安监站安全生产工作总结
 反恐防暴应急预案及措施小学反恐防暴应急预案
反恐防暴应急预案及措施小学反恐防暴应急预案
2024-02-12 5页
 覆铜板实习报告
覆铜板实习报告
2023-04-13 6页
 小班健康好玩的球创新游戏教案反思
小班健康好玩的球创新游戏教案反思
2024-01-03 5页
 版浙江新高考化学选考总复习检测:专题8 第二单元 溶液的酸碱性 Word版含解析
版浙江新高考化学选考总复习检测:专题8 第二单元 溶液的酸碱性 Word版含解析
2022-10-12 7页
 给教师的一百条新建议读后感
给教师的一百条新建议读后感
2023-07-08 3页
 软件工程试题与答案
软件工程试题与答案
2023-12-20 20页
 物业服务标准化管理示范文本全套
物业服务标准化管理示范文本全套
2023-04-06 272页
 基于单片机的便携式甲醛检测仪的控制系统设计
基于单片机的便携式甲醛检测仪的控制系统设计
2022-09-18 57页
 元明清瓷器鉴定知识要点
元明清瓷器鉴定知识要点
2023-10-04 26页
 人教版 小学7年级 数学上册有理数复习练习
人教版 小学7年级 数学上册有理数复习练习
2023-03-23 4页

