
未获得境外医疗器械上市许可的第二三类境外医疗器械首次注册
5页1、the party. Focus on honesty and self-discipline standards of four clean four consciousness, hold the work principle and various violations of party discipline punishment . 2. series spoke. To XI on ruling acting political XI General Secretary series important speech reading (2016 version) XI General Secretary important speech articles selected (leaders reading) and out poverty for basic textbook, in-depth learning understand XI General Secretary series important speech spirit and on Fujian work
2、of series important indicates spirit, understand on reform development stable, and Interior diplomatic defense, and rule party ruling army of important discusses, understand throughout which of Marx doctrine position views method, Grasp the true faith through the pursuit of, historical sense, sincere feelings for the people, pragmatic style of thought. Should pay attention to the overall grasp and master relationship and prevent fragmentation. Address learning series, attention should be paid ba
3、sed on persisting and developing socialism with Chinese characteristics to the theme, focuses on understanding comrade XI as General Secretary of the CPC Central Committee on governance of the new concept of new ideas and new strategies. (1) the deep understanding we are carrying out the great struggle of history with many new features that Chinas development is an important period of strategic opportunities and the same connotation. (2) to grasp a firm system of socialism with Chinese character
4、istics theory of confidence, confidence, confidence, winning complete the building of a well-off society and realize two 100 year goal dreams, China and the great rejuvenation of the Chinese nation. (3) understand the domestic and international situations, coordination of five in one overall layout and four comprehensive strategy. (4) a deep understanding of innovation, coordination, development of green, open, shared ideas, adapt to new normal, grasp, leading economic development, greater empha
《未获得境外医疗器械上市许可的第二三类境外医疗器械首次注册》由会员206****923分享,可在线阅读,更多相关《未获得境外医疗器械上市许可的第二三类境外医疗器械首次注册》请在金锄头文库上搜索。

人教版小学英语单词分类记忆汇总表excel版

人教版九年级全一册英语词汇

2019年开展垃圾分类的工作总结报告【五篇】

六年级英语绘本教案

外研版小学英语单词表全带音标(一年级起点1-12册)

最新国家开放大学电大投资学网络核心课形考网考作业及答案

初中人教版七年级下册生物复习提纲

高中英语语法大全高中英语语法系统全解word版

☆初中英语语法专项练习习题以及答案

初中英语常考近义词同义词辨析

高中物理选修3-5全套教案(人教版) (1)

英语绘本《WeatherReport》教学设计

防护功能平战转换设计专篇各专业

初中人教版七年级上册下册全册生物复习提纲21页 (1)
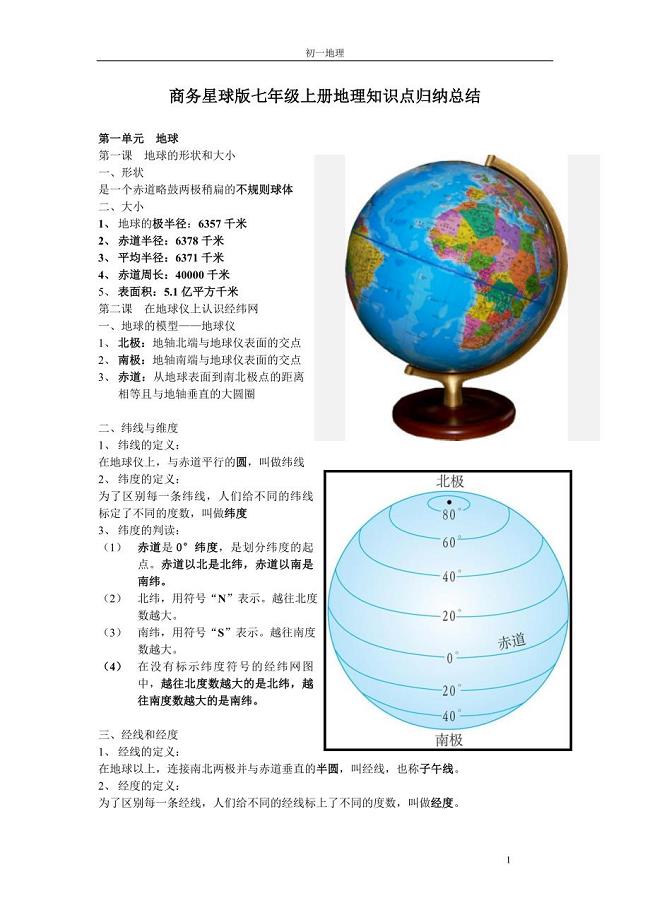
商务星球版七年级上册地理知识点归纳总结

初中人教版七年级上册下册全册生物复习提纲21页 (2)

高中高考语文作文词汇句型优美句子万能语句大全

中考地理选择题专项复习550题含答案
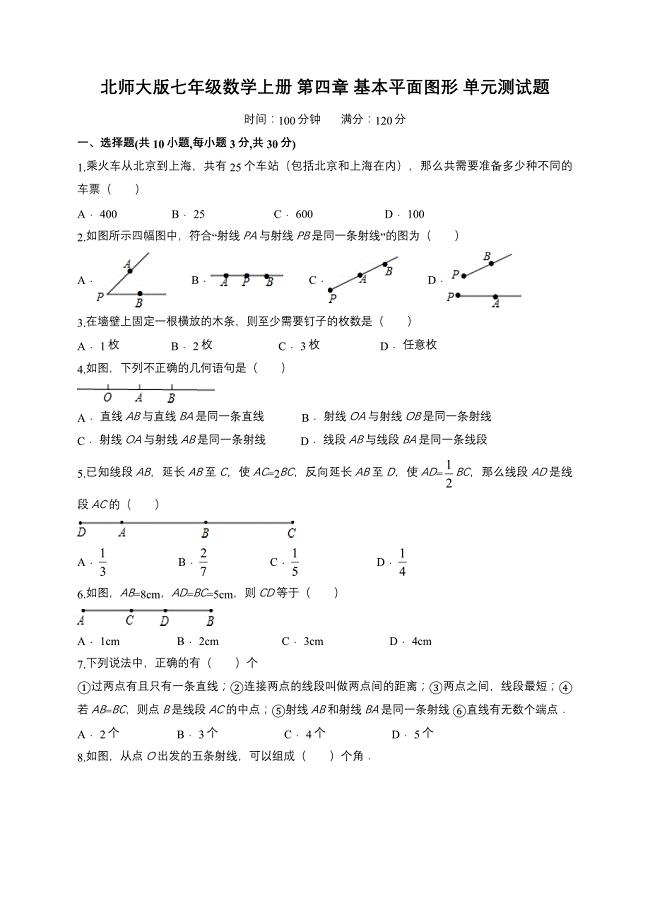
北师大版七年级数学上册第四章基本平面图形单元测试题含解析

人教版七年级数学上册第一章有理数单元检测题解析版
 小数数学题:简便计算11道练习题及参考答案A10
小数数学题:简便计算11道练习题及参考答案A10
2024-04-17 2页
 小数数学题:简便计算11道练习题及参考答案A7
小数数学题:简便计算11道练习题及参考答案A7
2024-04-17 2页
 小数数学题:简便计算11道练习题及参考答案A3
小数数学题:简便计算11道练习题及参考答案A3
2024-04-17 2页
 小数数学题:简便计算11道练习题及参考答案A5
小数数学题:简便计算11道练习题及参考答案A5
2024-04-17 2页
 小数数学题:简便计算11道练习题及参考答案A9
小数数学题:简便计算11道练习题及参考答案A9
2024-04-17 2页
 小数数学题:简便计算11道练习题及参考答案A6
小数数学题:简便计算11道练习题及参考答案A6
2024-04-17 2页
 小数数学题:简便计算11道练习题及参考答案A8
小数数学题:简便计算11道练习题及参考答案A8
2024-04-17 2页
 小数数学题:简便计算11道练习题及参考答案A1
小数数学题:简便计算11道练习题及参考答案A1
2024-04-17 2页
 小数数学题:简便计算11道练习题及参考答案A4
小数数学题:简便计算11道练习题及参考答案A4
2024-04-17 2页
 小数数学题:简便计算11道练习题及参考答案A2
小数数学题:简便计算11道练习题及参考答案A2
2024-04-17 2页

