
美国化学高考题
12页1、READ THESE INSTRUCTIONS FIRST Write your Centre number, candidate number and name on all the work you hand in. Write in dark blue or black pen. You may use a soft pencil for any diagrams, graphs or rough working. Do not use staples, paper clips, highlighters, glue or correction fl uid. DO NOT WRITE IN ANY BARCODES. Answer all questions. You may lose marks if you do not show your working or if you do not use appropriate units. A Data Booklet is provided. At the end of the examination, fasten all
2、your work securely together. The number of marks is given in brackets at the end of each question or part question. CHEMISTRY 9701/21 Paper 2 Structured Questions AS Core May/June 2012 1 hour 15 minutes Candidates answer on the Question Paper. Additional Materials: Data Booklet UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certifi cate of Education Advanced Subsidiary Level and Advanced Level This document consists of 11 printed pages and 1 blank page. Turn over IB12 06_9701_21/3RP
3、UCLES 2012 *1182281800* For Examiners Use 1 2 3 4 5 Total 2 9701/21/M/J/12 UCLES 2012 For Examiners Use Answer all the questions in the spaces provided. 1 Oxides are compounds which usually contain oxygen combined with one other element. Oxides are classifi ed as follows. acidic alkaline amphoteric basic (a) Using these terms only, complete the table to describe the oxides of the elements of the third period of the Periodic Table sodium to sulfur. Na2OMgOAl 2O3SiO2P4O10SO2Cl 2O7 acidic 4 (b) Giv
4、e the names of two elements from sodium to chlorine which form more than one oxide. . and . 1 (c) Sodium reacts with water. (i) Describe, as fully as you can, what you would see when a piece of sodium is reacted with water. (ii) Write an equation for the reaction of sodium with water. 4 3 9701/21/M/J/12 UCLES 2012 Turn over For Examiners Use (d) Sulfur dioxide is present in small, but signifi cant, amounts in the Earths atmosphere. (i) State one way by which sulfur dioxide enters the atmosphere.
《美国化学高考题》由会员n****分享,可在线阅读,更多相关《美国化学高考题》请在金锄头文库上搜索。

项目二财务管理价值观念

山东省安全生产风险分级管控与隐患排查治理信息化系统交流材料-2018.9.26

人教版高中地理必修3第一章地理环境与区域发展第二节《地理信息技术在区域地理环境研究中的应用》

第三章2房地产抵押贷款-固定利率抵押贷款
第八章工程质量法律制度

第25讲家庭电路与安全用电

餐厅点餐系统项目

项目7水箱水位控制

框架完整个人年度工作总结范文模板

科目名称-国土交通省

金融工程09课件

高校自主招生之结构化面试
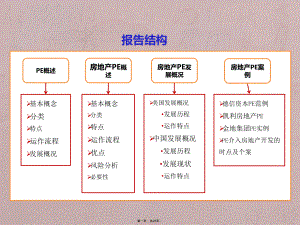
房地产私募股权投资基金(PE)专题研究.

房地产基础知识培训2012

第一章食品检测技术基础知识

第10章网站设计与建设综合实例

第5章尝试迷人的机器人项目机器人灭火项目

自考英语二unit3

企业人力资源管理师第六章劳动法与劳动关系管理

第三章市场营销宏观环境分析
 投标报价编制说明样板
投标报价编制说明样板
2023-04-04 16页
 南开大学22春《政治学概论》离线作业一及答案参考72
南开大学22春《政治学概论》离线作业一及答案参考72
2023-05-24 19页
 南开大学21秋《创业管理》平时作业2-001答案参考80
南开大学21秋《创业管理》平时作业2-001答案参考80
2024-01-29 18页
 21春《会计》软件实务在线作业二满分答案63
21春《会计》软件实务在线作业二满分答案63
2024-01-01 13页
 南开大学21春《税收制度与税务筹划》在线作业二满分答案32
南开大学21春《税收制度与税务筹划》在线作业二满分答案32
2022-10-29 16页
 中国医科大学2021年12月《药物代谢动力学》期末考核试题库及答案参考62
中国医科大学2021年12月《药物代谢动力学》期末考核试题库及答案参考62
2022-11-20 13页
 益都县图志06(古迹志)(150-168)
益都县图志06(古迹志)(150-168)
2023-08-09 20页
 中国教育在国际中的竞争力比较
中国教育在国际中的竞争力比较
2023-09-17 10页
 2022年3月《畜牧兽医法规》期末考核试题库及答案参考52
2022年3月《畜牧兽医法规》期末考核试题库及答案参考52
2022-08-27 13页
 医师定期考核表(全套)
医师定期考核表(全套)
2023-05-05 8页

